whatwomenmustknow.com
Drugs on Tap: What’s In Our Tap Water? Here’s a question to ponder. What happens to the hundreds of millions of prescription drugs and the over-the-counter medications that are swallowed daily? The answer: they go out through the plumbing. Being flushed down the toilet and into the sewage system, 90 per cent of every drug swallowed is either excreted, totally unchanged, or is

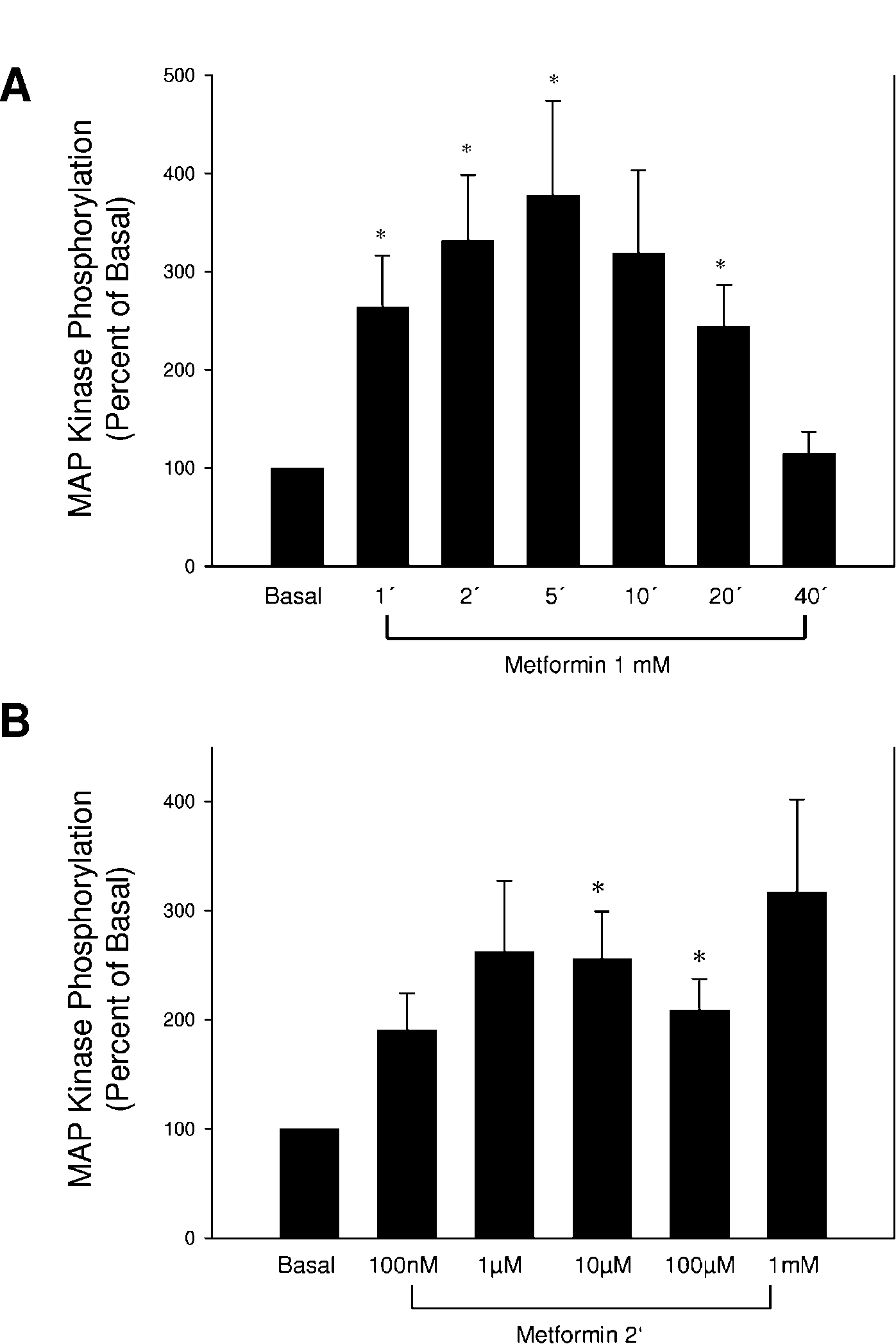 300 J KLEIN, S WESTPHAL and others · Direct effects of metformin on brown adipocytes
Figure 1 Metformin acutely activates p44/p42 MAP kinase. Fully differentiated brown adipocytes
300 J KLEIN, S WESTPHAL and others · Direct effects of metformin on brown adipocytes
Figure 1 Metformin acutely activates p44/p42 MAP kinase. Fully differentiated brown adipocytes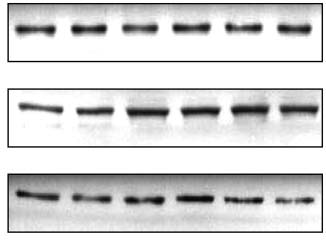
 Direct effects of metformin on brown adipocytes · J KLEIN, S WESTPHAL and others 301
By contrast to liver and muscle, relatively little is known
about direct metformin actions in adipocytes. In ratadipose tissue glucose uptake has been found to beenhanced (Matthaei et al. 1991, 1993) whereas in humanadipocytes no change has been described by metformintreatment (Pedersen et al. 1989, Ciaraldi et al. 2002).
Direct effects of metformin on brown adipocytes · J KLEIN, S WESTPHAL and others 301
By contrast to liver and muscle, relatively little is known
about direct metformin actions in adipocytes. In ratadipose tissue glucose uptake has been found to beenhanced (Matthaei et al. 1991, 1993) whereas in humanadipocytes no change has been described by metformintreatment (Pedersen et al. 1989, Ciaraldi et al. 2002).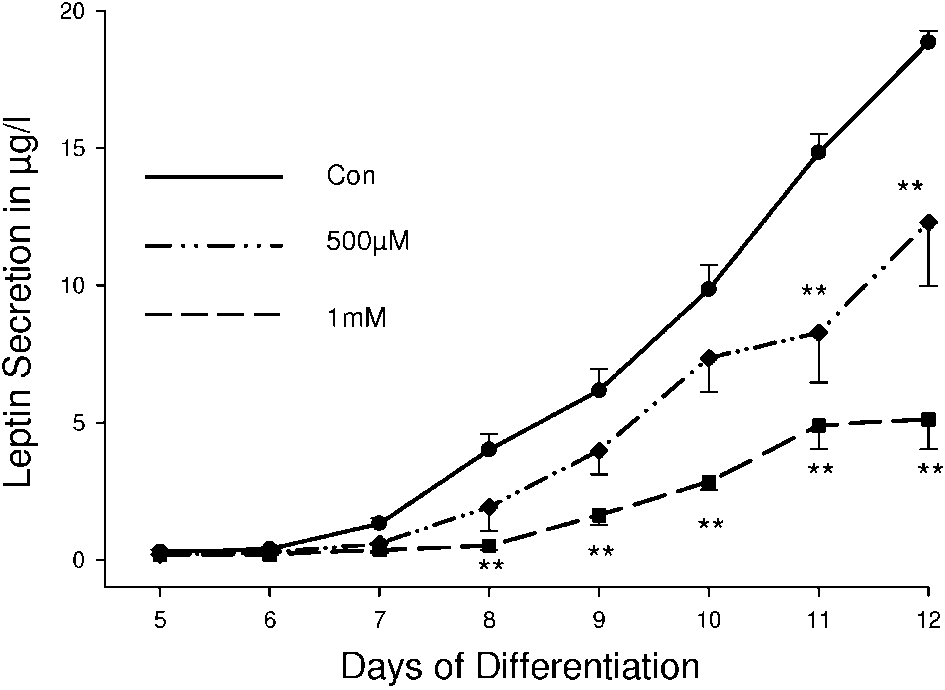 302 J KLEIN, S WESTPHAL and others · Direct effects of metformin on brown adipocytes
using whole cell lysis buffer containing 2 mM vanadate,10 µg/ml aprotinin, 10 µg/ml leupeptin, and 2 mMPMSF. Protein content of lysates was determined by theBradford method using the dye from Bio-Rad (Hercules,CA, USA). Lysates were submitted to SDS-PAGE andtransferred to nitrocellulose membranes (Schleicher andSchuell Inc., Keane, NH, USA). Membranes wereblocked with rinsing buffer (10 mM Tris, 150 mM NaCl,0·05% Tween, pH 7·2) containing 3% bovine serumalbumin (‘blocking solution’) overnight. Membranes werethen incubated in blocking solution for 1–2 h with theantibodies indicated. Protein bands were visualizedusing the chemiluminescence kit from Roche MolecularBiochemicals (Mannheim, Germany) and enhancedchemiluminescence films (Amersham Pharmacia Biotech,Freiburg, Germany).
302 J KLEIN, S WESTPHAL and others · Direct effects of metformin on brown adipocytes
using whole cell lysis buffer containing 2 mM vanadate,10 µg/ml aprotinin, 10 µg/ml leupeptin, and 2 mMPMSF. Protein content of lysates was determined by theBradford method using the dye from Bio-Rad (Hercules,CA, USA). Lysates were submitted to SDS-PAGE andtransferred to nitrocellulose membranes (Schleicher andSchuell Inc., Keane, NH, USA). Membranes wereblocked with rinsing buffer (10 mM Tris, 150 mM NaCl,0·05% Tween, pH 7·2) containing 3% bovine serumalbumin (‘blocking solution’) overnight. Membranes werethen incubated in blocking solution for 1–2 h with theantibodies indicated. Protein bands were visualizedusing the chemiluminescence kit from Roche MolecularBiochemicals (Mannheim, Germany) and enhancedchemiluminescence films (Amersham Pharmacia Biotech,Freiburg, Germany).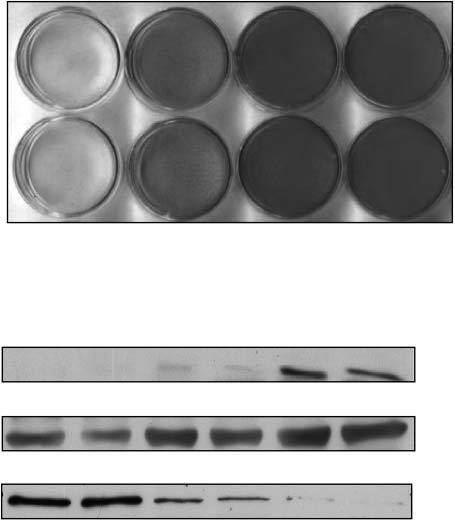
 Direct effects of metformin on brown adipocytes · J KLEIN, S WESTPHAL and others 303
Figure 4 The inhibitory effect of metformin on leptin secretion is not caused by
Direct effects of metformin on brown adipocytes · J KLEIN, S WESTPHAL and others 303
Figure 4 The inhibitory effect of metformin on leptin secretion is not caused by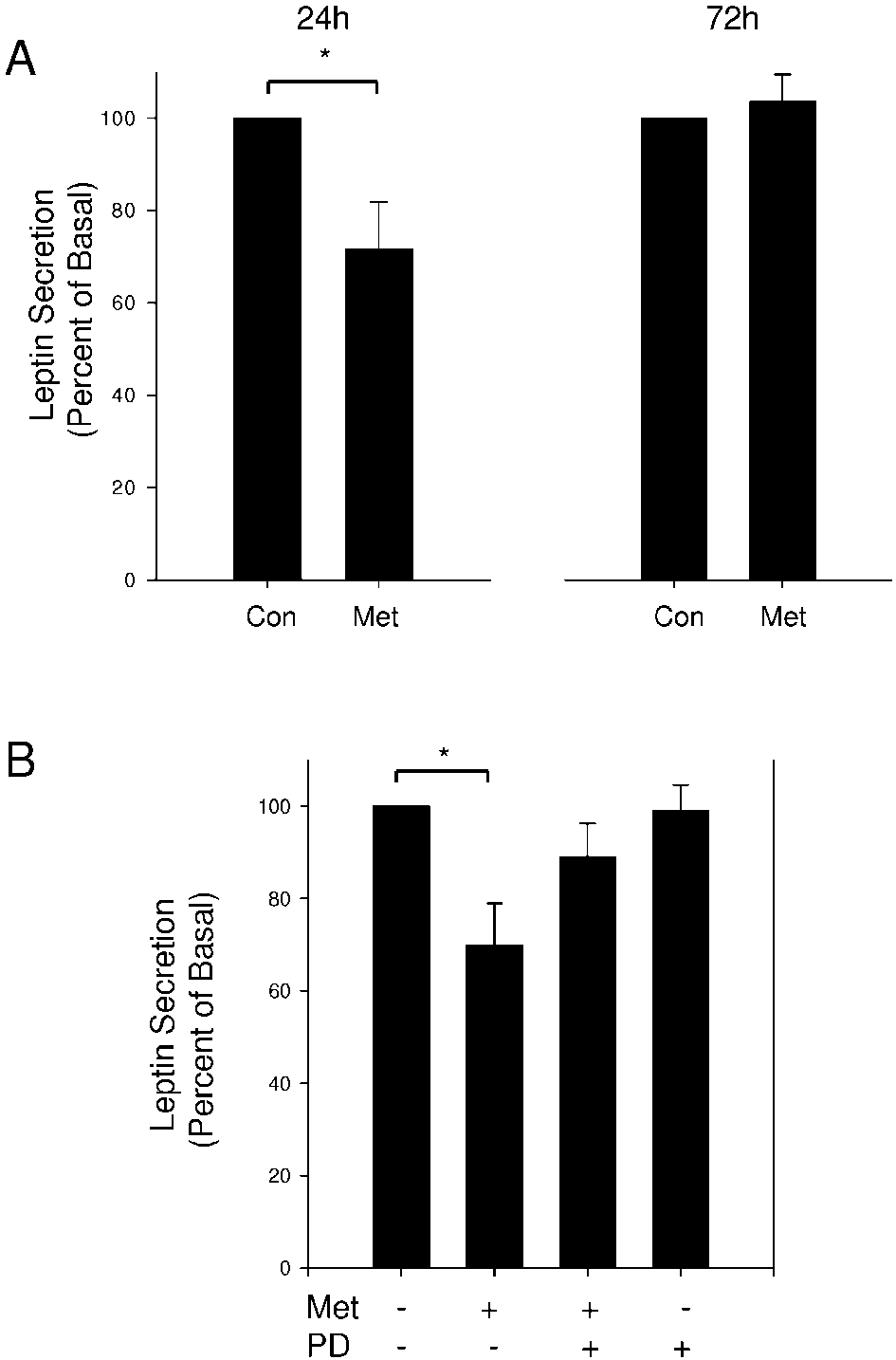 304 J KLEIN, S WESTPHAL and others · Direct effects of metformin on brown adipocytes
MAP kinase phosphorylation suggested an involvement ofthis signalling intermediate in the mediation of this effect.
304 J KLEIN, S WESTPHAL and others · Direct effects of metformin on brown adipocytes
MAP kinase phosphorylation suggested an involvement ofthis signalling intermediate in the mediation of this effect.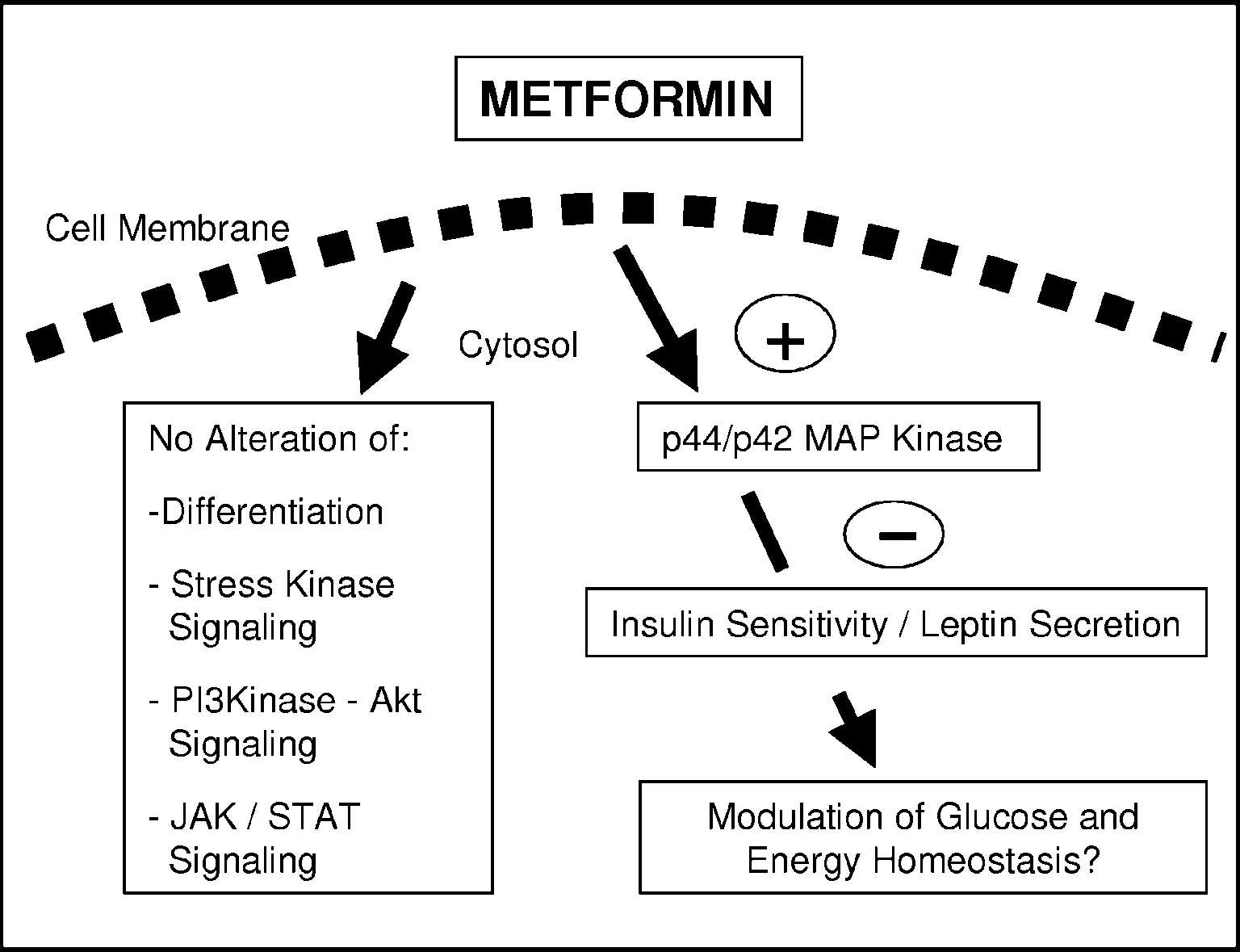 Direct effects of metformin on brown adipocytes · J KLEIN, S WESTPHAL and others 305
Figure 6 Metformin directly modulates adipocyte signalling and endocrine function. Metformin activates
Direct effects of metformin on brown adipocytes · J KLEIN, S WESTPHAL and others 305
Figure 6 Metformin directly modulates adipocyte signalling and endocrine function. Metformin activates