Doi:10.1016/j.neulet.2006.07.06
Neuroscience Letters 406 (2006) 289–292Effect of acute leg cycling on the soleus H-reflex and modifiedAshworth scale scores in individuals with multiple sclerosisRobert W. Motl , Erin M. Snook, Marcus L. Hinkle, Edward McAuley Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, 350 Freer Hall, Urbana, IL 61801, United States Received 21 April 20
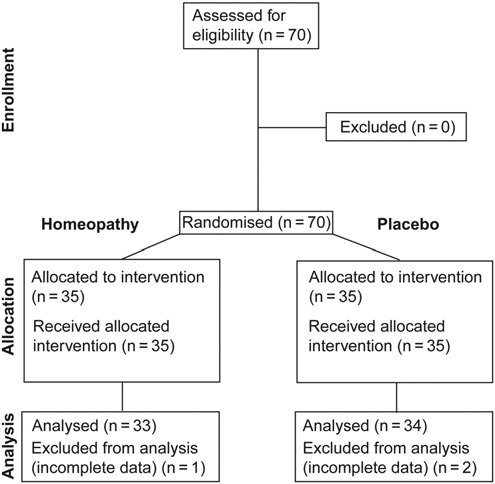 Homeopathic medicine has been used for about two centuries. Several studies describe its superiority above placebo.
Homeopathic medicine has been used for about two centuries. Several studies describe its superiority above placebo.