Microsoft word - texto la captura deslumbrante - alfredo torres.doc
La captura deslumbrante La exposición de dibujos de Clarina Vicens y Eduardo Vernazza es concebida, desde el guión curatorial, como una muestra de cámara. Usando ese carácter no sólo por la imposición de las salas, estrechas, techo bastante bajo, y, por su ausencia de aberturas, un tanto ensimismadas y propensas a la mirada cercana. El encuadre, entonces y de manera esencial, sur
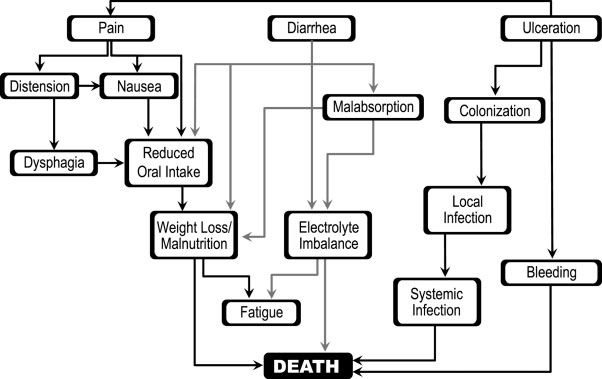 they will provide opportunities for intervention. In
els have continued to characterize oral and GI muco-
the future, it may be possible to predict an individual
sitis as a continuum of injury rather than biologically
patient’s risk of particular toxicities from radiother-
and clinically independent toxicities,23,27,28 new mod-
apy and from each different chemotherapy or combi-
els for the assessment of genetic risk for mucositis29
nation therapy. We recommend future research into
and the totality of impact for the condition23 may de-
mucotoxicity that focuses on identifying its generic
velop as research in this area progresses); 4) develop-
and tissue-specific causes, analyzing its genetic com-
ment of an epidemiological burden-of-illness study
ponents using genetic versus nongenetic controls,
by researchers from the Mucositis Study Group to
and establishing evidence-based grading for and
evaluate the impact of AM on patients after treatment
hierarchical characterization of mucotoxicity.
they will provide opportunities for intervention. In
els have continued to characterize oral and GI muco-
the future, it may be possible to predict an individual
sitis as a continuum of injury rather than biologically
patient’s risk of particular toxicities from radiother-
and clinically independent toxicities,23,27,28 new mod-
apy and from each different chemotherapy or combi-
els for the assessment of genetic risk for mucositis29
nation therapy. We recommend future research into
and the totality of impact for the condition23 may de-
mucotoxicity that focuses on identifying its generic
velop as research in this area progresses); 4) develop-
and tissue-specific causes, analyzing its genetic com-
ment of an epidemiological burden-of-illness study
ponents using genetic versus nongenetic controls,
by researchers from the Mucositis Study Group to
and establishing evidence-based grading for and
evaluate the impact of AM on patients after treatment
hierarchical characterization of mucotoxicity.