aisf.be
Arrêté ministériel établissant la liste des produits et méthodes interdites pour l'année 2013 A.M. 17-12-2012 M.B. 16-01-2013 Le Ministre des Sports de la Communauté française ayant en charge la lutte contre le dopage dans ses attributions, Vu le décret du 20 octobre 2011 relatif à la lutte contre le dopage, Vu l'arrêté du Gouvernement de la Communauté française
 Tropical Medicine and International Health
K. Schnippel et al. Costs of MDR-TB inpatient care
reported being resident in North West Province at admis-
72% of smear-negative patients had resistance to only
sion. 64% were unemployed. A large majority (n = 111,
first-line anti-TB drugs. In contrast, 53% of smear-posi-
83%) had a history of previous TB treatment. The mean
tive patients had resistance to one or more second-line
interval from collection of sputum for DST to laboratory
report MDR-TB diagnosis was 84 days; and from sputumcollection to hospital admission was 111 days. By defini-
tion, all patients were TB culture positive at diagnosis,with INH and RIF resistance confirmed by either DST or
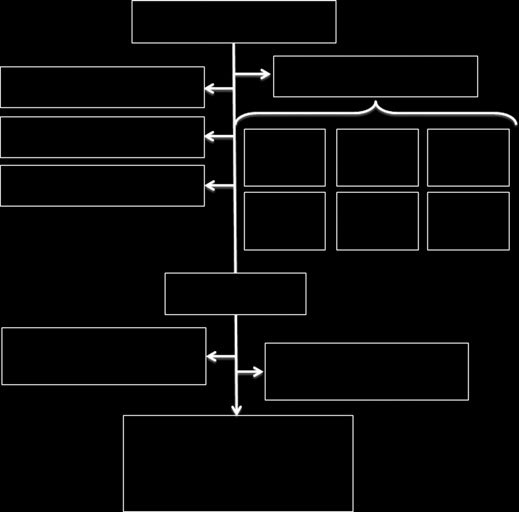
Of the 133 subjects enrolled in the study, 121 had com-
LPA or both. 56% of patients were smear positive at
plete information on outcomes and resource utilisation in
their medical records. This smaller sample of 121 is used
Of 133 enrolled study subjects, four did not have a
smear microscopy taken on admission and one patient’s
After 12 months, 98% of patients who were smear
sputum sample was rejected, leaving a total of 128 sub-
negative at admission and 80% of those who were smear
jects with known smear status at baseline. The sample
positive had been discharged upon culture conversion
was evenly divided between patients who were smear
(Table 2). Three patients, all of whom were all smear
negative and smear positive at hospital admission. Three-
positive at admission and resistant to one or more sec-
quarters of smear-negative patients and more than half
ond-line TB drugs, were still hospitalised at the end of
of smear-positive patients were HIV infected, of whom
12 months. Ten patients (8%) died while admitted, all of
43% were on ART at admission. Low body mass index
whom were HIV infected. Four (3%) absconded.
Tropical Medicine and International Health
K. Schnippel et al. Costs of MDR-TB inpatient care
reported being resident in North West Province at admis-
72% of smear-negative patients had resistance to only
sion. 64% were unemployed. A large majority (n = 111,
first-line anti-TB drugs. In contrast, 53% of smear-posi-
83%) had a history of previous TB treatment. The mean
tive patients had resistance to one or more second-line
interval from collection of sputum for DST to laboratory
report MDR-TB diagnosis was 84 days; and from sputumcollection to hospital admission was 111 days. By defini-
tion, all patients were TB culture positive at diagnosis,with INH and RIF resistance confirmed by either DST or
Of the 133 subjects enrolled in the study, 121 had com-
LPA or both. 56% of patients were smear positive at
plete information on outcomes and resource utilisation in
their medical records. This smaller sample of 121 is used
Of 133 enrolled study subjects, four did not have a
smear microscopy taken on admission and one patient’s
After 12 months, 98% of patients who were smear
sputum sample was rejected, leaving a total of 128 sub-
negative at admission and 80% of those who were smear
jects with known smear status at baseline. The sample
positive had been discharged upon culture conversion
was evenly divided between patients who were smear
(Table 2). Three patients, all of whom were all smear
negative and smear positive at hospital admission. Three-
positive at admission and resistant to one or more sec-
quarters of smear-negative patients and more than half
ond-line TB drugs, were still hospitalised at the end of
of smear-positive patients were HIV infected, of whom
12 months. Ten patients (8%) died while admitted, all of
43% were on ART at admission. Low body mass index
whom were HIV infected. Four (3%) absconded.