Doi:10.1098/rsbl.2005.0308
scientific interest may be partly attributable to theprevious consensus that the female orgasm has noclear role in reproduction. This view was challengedby research showing that the orgasm helped facilitatesperm retention (Further evidence for the orgasm’s reproductive rolecomes from studies linking it with the menstrual cycle(More recently, this was corroborated in other studiesshowing tha
 Human Reproduction, Vol.24, No.12 pp. 3196 – 3204, 2009
Advanced Access publication on October 3, 2009
ORIGINAL ARTICLE Reproductive epidemiology
Physical activity and fertility in women:the North-Trøndelag Health Study
S.L. Gudmundsdottir1, W.D. Flanders2, and L.B. Augestad1,3
1Human Movement Science Programme, Faculty of Social Sciences and Technology Management, Norwegian University of Science andTechnology (NTNU), NO-7491 Trondheim, Norway 2Departments of Epidemiology and Biostatistics, Emory University, Atlanta,GA 30345, USA
3Correspondence address. Tel: þ47 7359-1780; Fax: þ47 7359-1770; E-mail: liv.berit.augestad@svt.ntnu.no
background: Changes in the state of energy balance owing to changes in physical activity may affect the reproductive system. Weevaluated the association between physical activity (PA) and fertility and parity in healthy women.
Human Reproduction, Vol.24, No.12 pp. 3196 – 3204, 2009
Advanced Access publication on October 3, 2009
ORIGINAL ARTICLE Reproductive epidemiology
Physical activity and fertility in women:the North-Trøndelag Health Study
S.L. Gudmundsdottir1, W.D. Flanders2, and L.B. Augestad1,3
1Human Movement Science Programme, Faculty of Social Sciences and Technology Management, Norwegian University of Science andTechnology (NTNU), NO-7491 Trondheim, Norway 2Departments of Epidemiology and Biostatistics, Emory University, Atlanta,GA 30345, USA
3Correspondence address. Tel: þ47 7359-1780; Fax: þ47 7359-1770; E-mail: liv.berit.augestad@svt.ntnu.no
background: Changes in the state of energy balance owing to changes in physical activity may affect the reproductive system. Weevaluated the association between physical activity (PA) and fertility and parity in healthy women.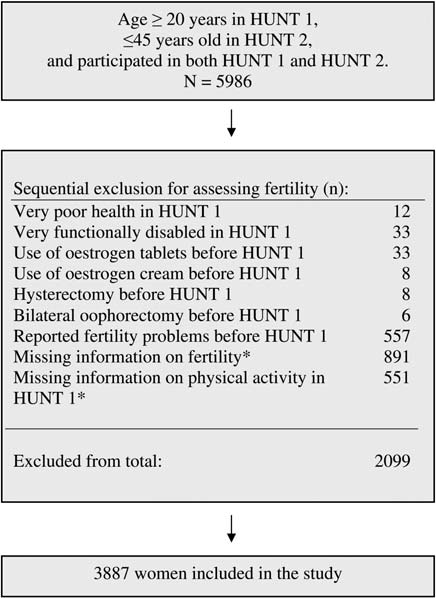 pregnancy loss than women who had reported not exercising. Moremoderate exercise together with weight loss has been found to be posi-tive in fertility treatment in obese women (Clark et al., 1995). The effectof PA on fertility may therefore be positive up to a certain level and havea negative effect above that threshold level of activity. A similar pattern issuggested for bone health: physically active individuals have been foundto have a lower risk of low bone mineral density and fractures, while therisk of stress fractures is increased with high-volume training (Kohrtet al., 2004). This may occur through the direct effects of activity on hor-mones which stimulate bone formation or indirectly via effects on estro-gen and menstrual function, a condition often coupled with negativeenergy availability in an interrelationship known as the female athletetriad (Nattiv et al., 2007).
pregnancy loss than women who had reported not exercising. Moremoderate exercise together with weight loss has been found to be posi-tive in fertility treatment in obese women (Clark et al., 1995). The effectof PA on fertility may therefore be positive up to a certain level and havea negative effect above that threshold level of activity. A similar pattern issuggested for bone health: physically active individuals have been foundto have a lower risk of low bone mineral density and fractures, while therisk of stress fractures is increased with high-volume training (Kohrtet al., 2004). This may occur through the direct effects of activity on hor-mones which stimulate bone formation or indirectly via effects on estro-gen and menstrual function, a condition often coupled with negativeenergy availability in an interrelationship known as the female athletetriad (Nattiv et al., 2007).