Cemseal e100cr
Cemseal E100CR High Performance Chemical Resistance Epoxy Coating Your Construction Chemical Partner The Product Cemseal E100CR is a 100% solid, pigmented, two component solvent-less epoxy coating. It is highly chemical resistant to acids, alkalis and a wide range of chemicals. Cemseal E100CR is designed for using as a concrete and steel protective coating as the thickness of 0.3mm-1.0mm
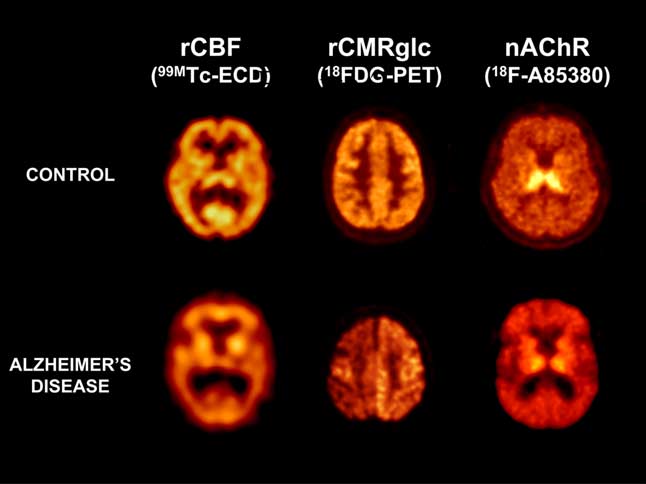 Structural neuroimaging techniques, such as computed tomogra-
phy (CT) and magnetic resonance imaging (MRI), are routinely
used in the clinical evaluation of AD patients.
Structural neuroimaging techniques, such as computed tomogra-
phy (CT) and magnetic resonance imaging (MRI), are routinely
used in the clinical evaluation of AD patients.